The time has come for pharmaceutical preparations to enter the international mainstream market
1 The Temptation of “Going Global†According to customs statistics, in 2007, the export of western medicine products in China increased at a rapid rate, and the export value hit another record high, reaching US$784 million, a year-on-year increase of 55.68%. However, according to relevant persons from the Medicare Chamber of Commerce, at present, Western medicines exported to Japan, South Korea, Australia, and other major markets are mostly products of “three-funded†enterprises, and are mainly exported through processing trade; while domestic-funded enterprises’ products are mainly exported. To Nigeria, Pakistan and other low-end markets. Due to the weak independent research and development capabilities and the mostly generic products of domestic pharmaceutical companies, the export of pharmaceutical preparations is mainly concentrated in Africa, Asia and other regions. "From the mainstream markets of Europe, the United States, and Japan, domestic-funded enterprises currently have very little benefit," the source said.
Yu Mingde believes that in the 643 billion US dollars global pharmaceutical market, the three major markets in Europe, the United States, and Japan account for 88% of the total market, which is the mainstream market in the world. "The three major market systems in Europe, the United States, and Japan are different from ours in the domestic market. The same generic pharmaceutical preparations in the three major markets can get a profit equivalent to 5 to 8 times the domestic market." More realistic is that the current domestic pharmaceutical companies Nearly half of the idle capacity, "a serious oversupply of production capacity" has been the shadow of many pharmaceutical companies lingering.
One side is the temptation of profit in the international mainstream market. One side is the overcapacity and vicious competition in the domestic market. It is not difficult for us to see that the state has emphasized the good intentions of the mainstream international markets for the preparation of pharmaceutical exports in the planning of the pharmaceutical industry and that “going out†has become the consensus of the industry.
“Internationalization is a breakthrough in the selection process.†Luo Guoliang, general manager of Dalian Meiluo Pharmaceutical Factory, told reporters that in 2005 the company took the lead through the Australian TGA certification, and in 2007 passed the TGA certification review. Mei Luo also received a number of overseas orders for OEM (OEM) in succession: 1.5 billion antipyretic analgesics from the United States; 120 million Ginkgo biloba extracts from Germany; and Latanoprost from Ellen, UK Eye drops and so on. "In the first half of this year, the production scale of our OEM solid formulations will increase to 3 billion tablets per year," said Luo Guoliang. According to sources, the ANDA filed by Dalian Metro in the United States (a new drug application filed with FDA for a non-patented drug submission) project may also be approved by the end of this year.
Yu Mingde believes that the reform and opening up has narrowed the gap between China's pharmaceutical production and the world. R&D, innovation and the implementation and upgrade of quality standards such as GMP, GCP, GLP, and GSP have brought us closer to the quality standards of the mainstream market. In Ming De's view, the time has come for China Formulations to go out.
2 who is playing the tide?
If we say that the international mainstream market is a blue ocean to be developed, then the first enterprise to eat crabs is undoubtedly the culmination of a wise and courageous business. Among them, there are many emerging companies such as Shenzhen Lijian and Zhejiang Rishengchang.
According to Ouyang Qing, general manager of Shenzhen Lijian Pharmaceutical, on July 21st, 2006, the company passed the German cGMP certification including four formulations of tablets, capsules, granules and sterile powder injections, cefuroxime and cephalosporins. A total of 12 specifications of powder injections of zoledillin, ceftriaxone and cefotaxime were obtained in the EU market. According to the relevant regulations of the European Union, the marketing license of the preparations is only issued to the legitimate enterprises in the EU. For overseas companies to sell their preparations to the EU market, they must establish local branches or through local partners. It is understood that the US FDA certification also has the same requirements.
Yu Mingde pointed out that under this premise, Chinese preparations must enter the European and American markets, and it is more feasible to acquire local companies, register branches, or find partners through other means. Among them, finding local partners is the easiest route for Chinese companies. . According to report, Haizheng Pharmaceutical Co., Ltd., Wuxi Kaifu Co., Ltd., and Shanghai Tian-Equal Co., Ltd., which have passed the EU certification, have all chosen this path: Hisun Pharmaceutical Co., Ltd. has passed the European Union's EDQM certification and has been designated as an international agent factory. Production rights; Shanghai Tianping has passed the UK's MHRA solid formulation certification and Australia's TGA water solvent certification, OEM production for the British Watts; Wuxi and Shenzhen Lijian passed the German cGMP certification, commissioned by the German company processing plant Identity enters the European market.
The efforts made by these companies to “go global†are obvious to all, and choosing a good foreign partner is also an essential part of the formulation's export. In Ming De's 30 years of pharmaceutical industry management career, there have been many opportunities to communicate with foreign companies. He told reporters that foreign policies, regulations, operational procedures and even humanities and traditions all have "too much difference" with China. There are also many segments between major markets. He believes that for Chinese pharmaceutical companies that have just “tested for waterâ€, in the reality that there are no sales channels in foreign countries, although OEM and commissioned forms are expedient, if they are in the future, they will be able to do a better job in this area. You can talk about mergers and acquisitions, register your own company, and support your own brand.
Zhejiang Rishengchang quickly passed the FDA certification as a representative example of “learning to use forceâ€. In 2004, Fu Longyun established Zhejiang Rishengchang Pharmaceutical Co., Ltd., at the time, Rishengchang's self-developed compound polymyxin B ointment had already applied for domestic clinical trials in 2003. In 2006, the company obtained a pharmaceutical production license. In early 2007, a well-known drug distributor in the United States took the initiative to find Rishengchang to produce ointment products for its North American market. After full inspections, research, and several face-to-face exchanges, the company quickly reached a long-term cooperation agreement with Rishengchang. In the same year, Rishengchang's compound polymyxin B ointment passed the FDA registration and certification. In November 2007, the first batch of compound polymyxin B ointment valued at US$300,000 was sent to the United States from the Rishengchang production base in Dongyang, Zhejiang.
A person from a medical consulting company told the reporter that similar generic drugs have low R&D investment, and they can apply to the US FDA for a simplified new drug application (Abbreviated New Drug Application, ANDA) only after they have done a bioequivalence test. Raw materials exports more profits.
3 Breakthrough barriers After China's accession to the WTO, the global pharmaceutical market transfer production and commissioning processing business has been expanding. Many multinational companies such as Europe, the United States, Japan, etc. have transferred their raw material medicines and intermediate products to Chinese enterprises for production. “The transfer of production to local companies has tampered with international courses, environmental protection concepts, quality standards, and patent protection courses. This has greatly improved our competitiveness and international status.†Yu Mingde believes that behind OEM orders and profits, The docking of international standards and breakthrough in product quality are the thresholds for China's preparations to be exported.
The transformation of such raw material drug exporters as Hisun and Huahai has brought many thoughts to the industry.
Ouyang Qing believes that in terms of hardware, the gap between the access standards of the mainstream market in China and the EU is not large, and the gap is mainly in software management, such as verification, risk assessment, deviations and other details. "International certification focuses more on verification, emphasizing the normative and controllability of the system."
Ouyang Qing said. Yu Mingde emphasized that in addition to the certification of preparations abroad, there are joint certifications for APIs to ensure the stability of product quality. In addition, China's new drug GMP standards are close to EU standards, indicating that the thresholds and specifications of the local pharmaceutical industry are continuously in line with international standards.
With regard to how to choose the breakthrough point for the export of preparations, the forerunners have their own experience. According to Ouyang Qing, Shenzhen Lijian's predecessor was engaged in the export of bulk drugs, and the excellent product quality won a long-term order for a German company. When Lijian decided to increase the production of high-value-added formulations, its German partners are also considering moving their formulation OEMs from France and Italy to the low-cost market. The two sides hit it off. "The German partners played a vital role in Li Jian's acquisition of EU certification." Ouyang Qing did not say anything about it. He believes that many domestic third-party companies that carry out relevant certification consultations are more involved in the introduction of processes and texts, and lack the emotional experience of field operations. “If there is an overseas partner who is very familiar with the export destination country certification system, it will undoubtedly Do more with less."
Fu Longyun also admitted that the success of Rishengchang not only benefits from the management team of senior R&D personnel, but also the long-term business experience and extensive resources of the famous US pharmaceutical sales company in the Americas. The reporter learned that the entire FDA certification process was performed by Rishengchang's management personnel and did not use any translators or consultants. “This ensures that we have full control over the certification process and high efficiency.†Fu Longyun said.
In fact, one of the tricks of Rishengchang's rapid certification is product selection. Compound polymyxin B ointment is an OTC product in the United States. The US FDA has detailed provisions on the quality, specification, and labeling of OTC products. The product is widely used, and its efficacy and safety have been generally recognized. Rishengchang, as China's first imitation, adopted the FDA standard when doing domestic related certification, which laid a good foundation for the future of international cooperation.
“In terms of formulation exports, China’s main competitor is India.†In Luo Guoliang’s view, the key to the Chinese pharmaceutical industry’s participation in the global market competition is to verify system docking, employees’ language skills, product selection, and research and development capabilities. In addition, management must have a strategic grasp of the future, abandon opportunism, and do long-term planning. It is understood that Dalian Meiluo has invested a total of 100 million yuan in the export of pharmaceutical preparations. Although orders have been received overseas, no returns have yet been generated.
However, people who are new to Blue Ocean are optimistic about the future. "Do not underestimate the mainstream market demand for Chinese generic pharmaceutical preparations. They expressed great interest and tolerance to Chinese companies."
Ouyang Qing said. Yu Mingde believes that the “going out†of Chinese preparations is definitely a future trend. In the future, more and more domestic companies will be allowed to enter the mainstream market. He also reminded enterprises that the price and variety of the commissioned processing business in the international market will also generate new competition. The advantage of Chinese manufacturing lies in the low cost and high quality. The first company that undertakes external processing business is usually the highest profit, followed by Corporate profits will gradually decrease.
1. Introduction of Waste Motor Oil Distillation Plant
Waste Motor Oil Distillation Plant ,is the new technology which can refine the waste motor oil into base oil(which can be made into diesel and gasoline after processed by our catalyst). The oil quality is better than the original normal pressure distillation technology, which show on purity ,transparence, lightness .this technology will do deodorization and destinke process to the raw material oil automatically by "dry type" vacuum pressure distillation method. With the vacuum distillation technology, the distillation temperature is considerably reduced, and the oil output will higher 5%-10% compared with original normal pressure distillation technology. It makes more profits to the enterprise virtually.
2. Raw material which can be used
a. Waste oil .example: waste diesel, waste oil residue etc.
b. tire/rubber oil
c. plastic oil
d. crude oil
e. waste engine oil
f. waste motor oil
g. waste lube oil
h. waste transformer oil
i. underground oil
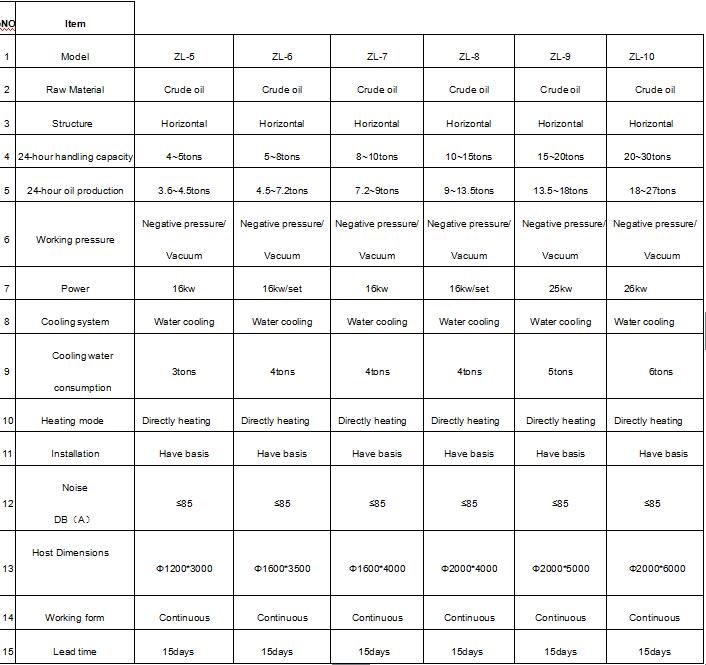
3. Models of waste motor oil distillation plant
4. Installation: We will be in charge of arranging our engineer to go to your place to guide the installation and train your workers how to operate the waste motor oil distillation plant ,and buyer will be in charge of the food, accommodation and round air tickets.
5.Waste Motor Oil Distillation Plant Exporting Experience:
|
America: |
Brazil, Canada, Colombia, USA, |
|
Middle East: |
Dubai, Iran, Jordan, Saudi Arabia, Turkey |
|
Europe: |
Albania , Bosnia and Herzegovina |
|
Asia: |
Afghanistan, India, Malaysia, Pakistan, Philippines, South Korea, Vietnam, Myanmar |
|
Africa: |
Ghana, Mozambique, Zambia |
Waste Motor Oil Distillation Plant
Waste Motor Oil Distillation Plant,Used Motor Oil Regeneration Plant,Waste Oil Recycling Diesel Plant,Diesel Oil Distillation Plant
Shangqiu Sihai Energy Technology Co., Ltd , https://www.sihaienergy.com